

2022-09-01
On September 1, 2022, Shanghai BRL Medicine Inc. (hereinafter referred to as "BRL Medicine") announced the cooperation with East China Normal University and the First Affiliated Hospital of Zhejiang University School of Medicine's non-viral fixed-point integrated CAR-T technology (Quikin CART®) research results were officially published in the top international academic journal Nature on August 31 . It is worth mentioning that this is the first CAR-T research result published in the top journal Nature in China .

Nature published
Quikin CART® is a non-viral site-specific integration CAR-T platform with independent intellectual property rights built by BRL Medicine. It can obtain genome-specific site-integrated CAR-T cell products through one-step preparation without the use of viral vectors, with low cost , short preparation time, simple process, high safety and effectiveness. In May 2020, in the world's first "PD1-knockout non-virus site-directed integration of CD19-CART cells in the treatment of relapsed and refractory non-Hodgkin's lymphoma clinical trial" carried out by the First Affiliated Hospital of Zhejiang University School of Medicine, the product embodies In addition to excellent clinical safety and efficacy, the first patient treated has been in complete remission (CR) for more than 2 years.
Break the shackles of relapse and refractory treatment, and ignite hope for more patients
Non-Hodgkin's lymphoma is a hematologic malignancy that originates in lymphoid tissue, accounting for 80%-90% of all lymphomas. Although patients get remission after initial treatment, they often relapse later. Although CAR-T products have been approved for the clinical treatment of relapsed and refractory non-Hodgkin's lymphoma, the overall efficacy is still limited. The PD-L1/PD1 signaling pathway is an important immune checkpoint that inhibits T cell function. Many studies have reported that PD1 knockout can effectively enhance the function of CAR-T cells.
Therefore, BRL Medicine developed a CD19-targeting non-viral PD1 - CAR-T product (BRL-201) using the Quikin CART® platform. In preclinical studies, compared with CAR-T cells infected with lentivirus and combined with gene editing technology to knock out PD1, BRL-201 showed stronger, With a longer-lasting killing effect, the survival rate of mice was significantly improved. Mechanism studies have shown that Quikin CART® technology itself can significantly increase the proportion of memory T cells, and the down-regulation of PD1 expression can effectively enhance the anti-tumor immune function of T cells. In the clinical trial of BRL-201 in the treatment of relapsed and refractory non-Hodgkin's lymphoma, after 8 patients received treatment, no CAR-T-related neurotoxicity and cytokine storm above grade 2 were observed, proving that BRL- 201 has an excellent clinical safety profile. According to the test results, after reinfusion, CAR-T cells can rapidly expand and last for a long time in patients. After receiving treatment, 87.5% of patients achieved complete remission (CR), and all patients responded to treatment, objectively. The response rate (ORR) was 100%. So far, the longest cancer-free survival period of patients receiving this CAR-T therapy has exceeded 2 years, and they are still in a state of complete disease remission. It is worth mentioning that BRL-201 has shown good curative effect both in the treatment of patients with tumors with high PD-L1 expression and in the condition of low CAR-T cell reinfusion dose and positive rate, proving that It has strong tumor killing ability.
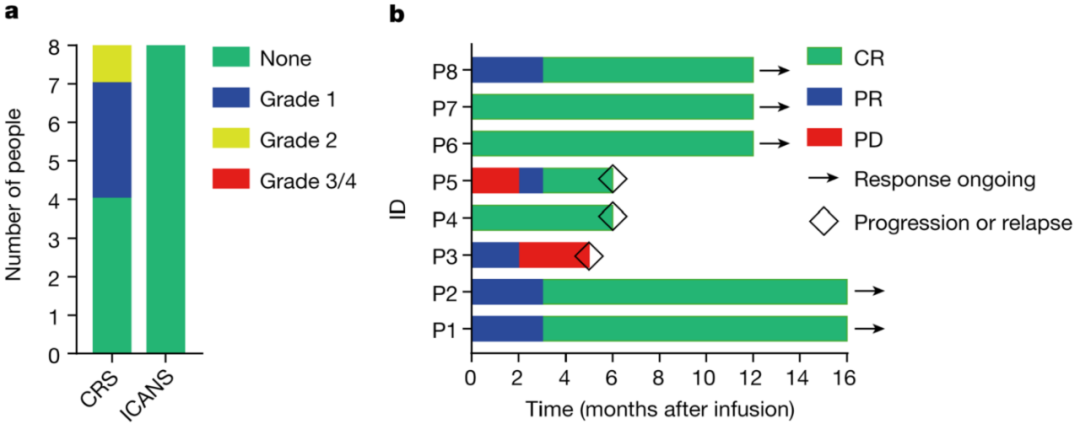
BRL-201 has demonstrated excellent safety and efficacy in clinical practice
It is worth mentioning that this achievement has also been highly praised by Professor Justin Eyquem of the University of California and Victoria Aranda, senior editor of Nature : "Comprehensive and systematic preclinical research has successfully developed a non-viral site-specific integrated CAR-T therapy, and reported the first The results of a clinical trial of PD1 down-regulation site-specific integrated CAR-T cells. The researchers observed a high proportion of complete tumor remission rates in clinical treatment, and no serious toxic side effects were found. This encouraging result shows that this CAR-T therapy has excellent clinical safety and efficacy. The researchers also proved the feasibility of non-viral site-directed integration of T cell therapy in clinical application. This technological innovation provides a basis for more gene-targeted modification of CAR-T therapy in the future The development has laid a solid foundation and played an important role in promoting the development of the field."