

2021-07-20
Recently, the clinical study on the safety and efficacy of γ-bead protein reactivated autologous hematopoietic stem cell transplantation for the treatment of beta-thalassemia major conducted by Chinese People’s Liberation Army Lianqin Guarantee Budui No.923 Hospital and BRL Medicine Inc. This is the first project in Guangxi to cure β-thalassemia major through gene therapy, and so far three children have been freed from blood transfusion with the help of gene editing technology. Next, this research project will further expand the target age to 35 years old, which is expected to benefit the "oldest group" in the field of thalassemia transplantation - older and hopelessly mismatched patients.
The clinical trial is a multi-center clinical trial, which has been conducted in Xiangya Hospital Central South University and Chinese People’s Liberation Army Lianqin Guarantee Budui No.923 Hospital. On July 22, 2020, BRL Medicine reported the initial results of the project in collaboration with Xiangya Hospital of Central South University. Two patients treated have been freed from blood transfusion after the treatment, and the follow-up period has now exceeded one year, and the total hemoglobin is still maintained within the normal range. Through a multi-center clinical trial, the gene therapy provided by BRL Medicine has cured five children with severe beta-hemoglobinemia, further confirming the feasibility and efficacy of the technology. At present, BRL Medicine is pushing forward with the new drug filing process and strives to bring the new drug to the market as soon as possible for the benefit of a wider group of patients with severe beta thalassemia.
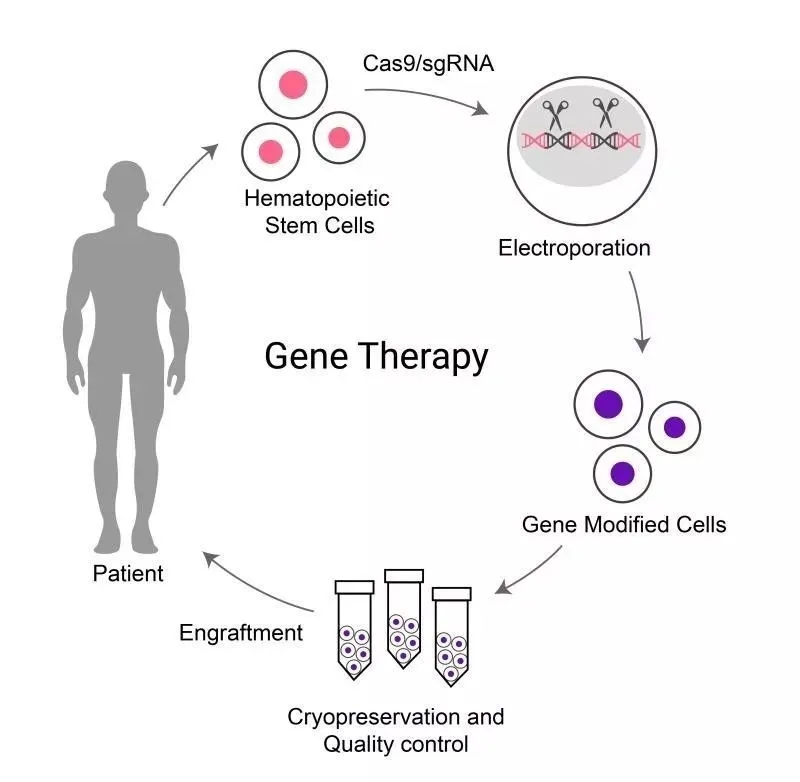
Principle of Gene Therapy
Focusing on the genetic field, BRL Medicine continues to break through and upgrade
In previous research, a team of BRL Medicine scientists has continued to make a series of breakthroughs in the field of gene editing tool development and gene therapy for thalassemia. This project uses gene editing technology to reopen the expression of fetal gamma bead protein to replace the defective beta bead protein, which is likely to be one of the treatment options to cure patients with thalassemia.
The pharmaceutical field represented by gene therapy and cell therapy is facing unprecedented opportunities and is the major breakthrough direction in the pharmaceutical field in the next decade. BRL Medicine is committed to developing innovative gene therapy drugs. Since its inception, BRL Medicine has established an international strategy of "base in China, layout globally, and benefit globally" to meet the rapidly growing medical market in China and globally, and to bring good news to patients who are in urgent need of revolutionary gene therapy. BRL Medicine;has differentiated competitive advantages in product line selection and technology platform, for the core pipeline product - gene therapy for β-deprivation. Overcoming the industry barriers, BRL Medicine; has both comprehensive mastery of gene editing technology and in-depth understanding of hematopoietic stem cell editing strategy. In turn, the process has been continuously optimized to solve the key problems of gene editing hematopoietic stem cells in the treatment process.