

2025-04-18
April 18, 2025 – Shanghai BRL Medicine Inc. (BRL Medicine), a company focused on gene and cell therapy, announced that BRL Medicine, jointly with the research team led by Professor Mingyao Liu and Professor Bing Du from East China Normal University, has published a review article titled "CAR-T therapy in solid tumors" in the prestigious international journal Cancer Cell. This review systematically summarizes the breakthrough progress of CAR-T cell therapy in the field of solid tumors, deeply analyzes current challenges, and proposes multi-dimensional innovative strategies. This review provides important directions for global solid tumor immunotherapy research, marking a critical step in the full-scale advancement of CAR-T technology into solid tumors.

Breakthrough Achievements: From Bench to Bedside, Dawn of Solid Tumor Treatment
While CAR-T therapy has created "miracle cures" in hematological malignancies, it still faces significant challenges in solid tumors. In this review, the research team first compiled global clinical data for various solid tumors and elaborated on the breakthrough progress achieved by CAR-T cells targeting different antigens in treating solid tumors:
(1) Lung Cancer: Compared to traditional treatments, EGFR-targeting CAR-T cells extended the median overall survival of patients to 15.63 months.
(2) Liver Cancer, Gastric Cancer: After treatment with GPC3-CAR-T and CLDN18.2-CAR-T respectively, a 90% disease control rate was achieved.
(3) Prostate Cancer: After PSMA-CAR-T treatment, patient Prostate-Specific Antigen (PSA) levels decreased by over 95%.
(4)Ovarian Cancer, Glioma: After treatment with MSLN-CAR-T and GD2-CAR-T respectively, significant efficacy was observed with 70.2% (6-month survival rate) and 83.3% (12-month survival rate).
Although these innovative CAR-T therapies have shown potential in a subset of solid tumor patients, their overall clinical efficacy仍需提升 needs improvement. The team then analyzed the challenges facing CAR-T cell therapy in solid tumors, highlighting Four Major Bottlenecks Requiring Breakthroughs:
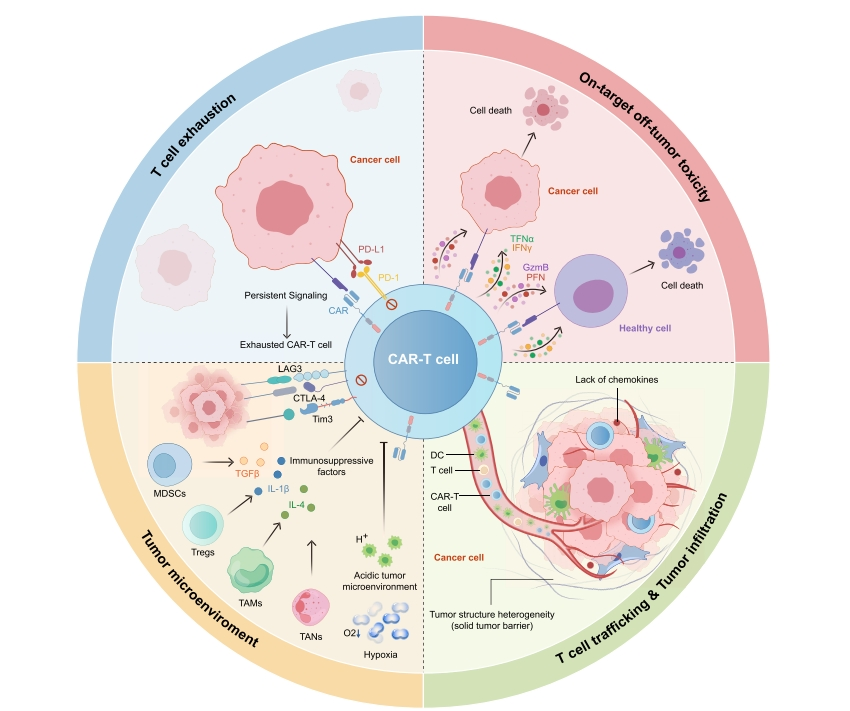
Figure 1. Challenges for CAR-T Therapy in Solid Tumors
Finally, the research team summarized progress in optimizing CAR-T cell therapy for solid tumors, systematically proposing 5 Major Innovative Upgrade Strategies to make CAR-T therapy more precise and safer:
(1) Smart CAR Design
Introduce "Dual-targeting" technology (e.g., GD2+B7-H3) to reduce off-tumor toxicity risk.
Integrate CAR and TCR technologies to develop novel receptors like "STAR-T", enhancing tumor recognition sensitivity.
(2) Enhance CAR-T Cell Function
Overexpress factors like IL-10, FOXO1 to enhance persistence and cytotoxicity.
lUse gene editing to knock out exhaustion-related genes (e.g., NR4A), prolonging CAR-T survival.
(3) Revolutionize CAR-T Manufacturing Processes
"Off-the-shelf" universal CAR-T enables immediate use, significantly shortening preparation time and reducing costs.
Non-viral site-specific integration technologies modify cells, improving safety by avoiding gene insertion risks.
(4) Disrupt the Tumor Immune Microenvironment
Equip CAR-T with "homing signals" (e.g., IL-7/CCL19) to guide deep tumor infiltration and enhance killing.
Engineer CAR-T to secrete LIGHT, heparinase to disrupt stromal barriers or remodel aberrant tumor vasculature.
(5) Improve CAR-T Treatment Safety
Design and engineer dual-targeting CAR-T cells to mitigate on-target, off-tumor toxicity.
Incorporate "Safety Switches" to promptly control side effects and block key Cytokine Release Syndrome (CRS) pathways.
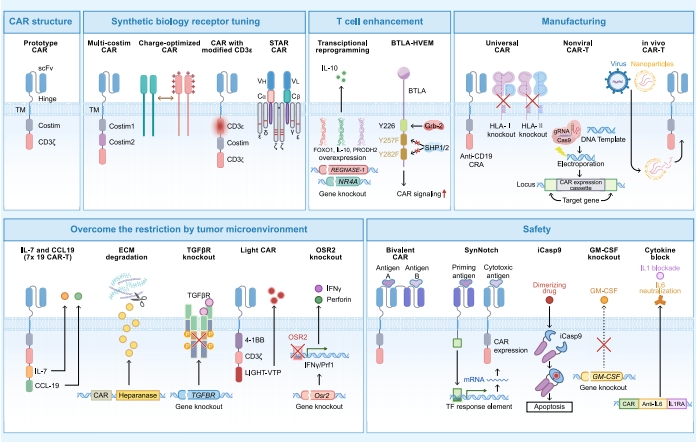
Figure 2. Strategies for Overcoming Solid Tumor Barriers
Professor Mingyao Liu, founder of BRL Medicine and co-author of the review, stated: "With the deep integration of gene editing, synthetic biology, and clinical translation, CAR-T has the potential to become the 'ultimate weapon' against solid tumors."
As a globally leading cell and gene therapy company, BRL Medicine has also achieved multiple breakthroughs in cell therapy for solid tumors. It has developed the proprietary enhanced T cell platform (HyperTCell®), which aims to overcome the worldwide challenge of solid tumor treatment through genetic modification of T cells. In January 2025, BRL Medicine's research team also published a study in Molecular Therapy, developing an innovative "GITRL-PSMA-CAR-T" cell that not only directly enhances the anti-tumor activity of CAR-T cells but also promotes their persistence within the solid tumor microenvironment, further improving CAR-T efficacy against solid tumors and potentially accelerating the development and application of CAR-T therapies for solid tumors. Undeniably, with the deepening understanding of the tumor immune microenvironment and T cell function regulation mechanisms in recent years, the engineering of CAR-T cells has become more systematic and diversified, leading to continuous breakthrough progress in the clinical treatment of solid tumors. The era of CAR-T for solid tumors has arrived.
Cancer Cell Review Article Link: https://doi.org/10.1016/j.ccell.2025.03.019