

2025-05-13
May 13, 2025 – Shanghai BRL Medicine Inc. (BRL Medicine), a company focused on gene and cell therapy, announced today the publication of a clinical research paper in the international academic journal Cell Research on May 8, titled "Allogeneic anti-CD19 CAR-T cells induce remission in refractory systemic lupus erythematosus". This work was completed in collaboration with Professor Hongyan Tong from the Department of Hematology and Professor Lin Jin from the Department of Rheumatology and Immunology at The First Affiliated Hospital, Zhejiang University School of Medicine, and a research team led by Professor Mingyao Liu and Professor Bing Du from the School of Life Sciences, East China Normal University. This represents the world's first reported successful case of using CRISPR/Cas9 gene-edited allogeneic anti-CD19 CAR-T cells (TyU19) to treat relapsed/refractory Systemic Lupus Erythematosus (SLE). The results indicate that TyU19 cells can achieve rapid and deep clinical remission in SLE treatment while demonstrating excellent clinical safety, opening a new paradigm for treating autoimmune diseases.
Cell Research Publication on May 8, 2025
TyU19 Technical Breakthrough Addresses Core Challenges of Allogeneic CAR-T Therapy
Systemic Lupus Erythematosus (SLE) is a systemic autoimmune disease characterized by aberrant B cell activation and autoantibody production, for which traditional immunosuppressants have limited efficacy in some patients. Although autologous CAR-T therapy has shown preliminary success in SLE, its personalized manufacturing involves long cycles and high costs. Allogeneic CAR-T cells, with advantages like homogeneity and immediate availability, present a promising alternative. However, their broad clinical application has been limited by risks of Graft-versus-Host Disease (GVHD), host rejection, gene editing-related genotoxicity, and infection risks from excessive immunosuppression.
In this Investigator-Initiated Trial (IIT), the research team evaluated the safety and efficacy of allogeneic CD19-targeting CAR-T cells (TyU19) in refractory SLE. BRL Medicine's TyU19 utilizes cells from healthy donors and employs a sophisticated multi-gene, selective gene-editing strategy for multiplex precision editing of donor T cells (knocking out TRAC, HLA-A, HLA-B, CIITA, and PD-1 genes), aiming to eliminate the risks of host rejection and GVHD at their root.
Between September 2023 and September 2024, the study enrolled four female patients (aged 22-24) with relapsed/refractory SLE. Their baseline SELENA-SLEDAI scores were 14-26, all had multi-system involvement (some with a history of lupus cerebritis), and were unresponsive to conventional immunosuppressants and biologics. Following TyU19 cell infusion, clinical results showed:
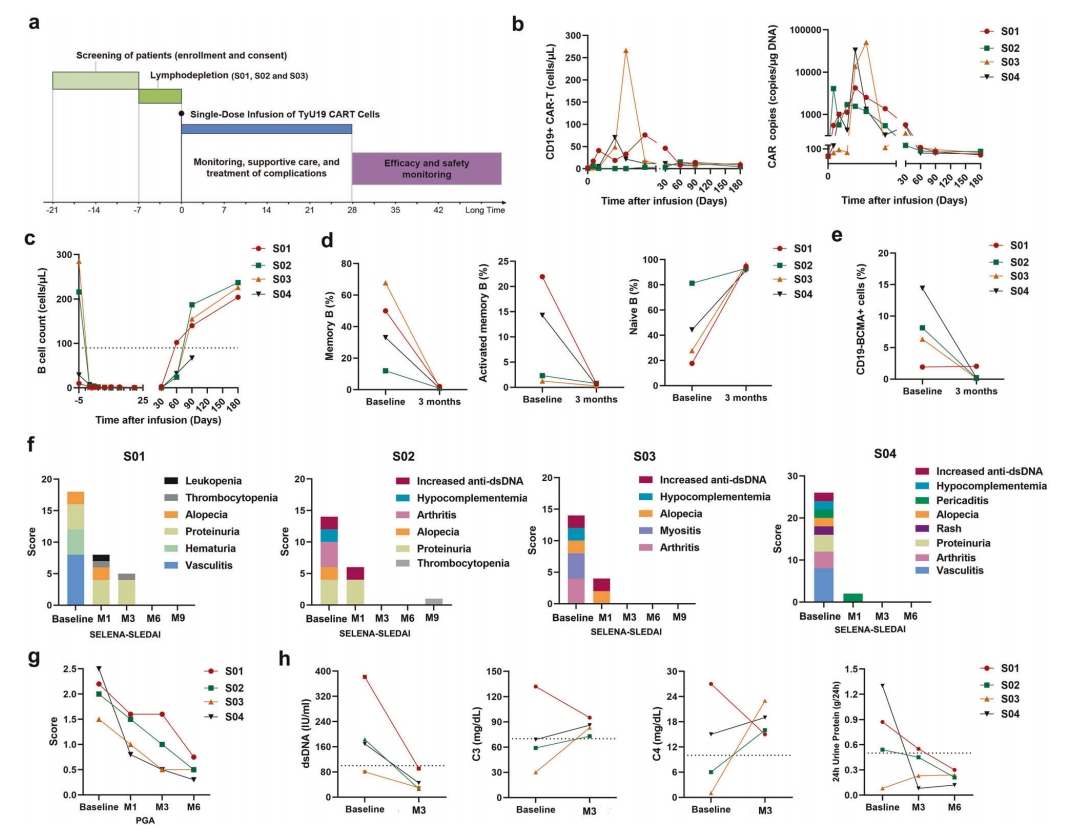
Clinical Response and Immune Reconstitution with TyU19 Treatment for SLE
About TyU19
TyU19 is a novel allogeneic universal CAR-T product targeting CD19, developed based on BRL Medicine's proprietary universal cell platform (TyUCell®). It utilizes multiplex gene editing technology to enable immune evasion and functional optimization of allogeneic T cells, circumventing the risks of GVHD and Host-versus-Graft (HVG) reaction at the source. This successfully overcomes the critical technical barrier in allogeneic universal CAR-T development: patient immune system rejection of allogeneic cells. It ensures the safety and efficacy of the cell product, truly realizing the universal application of immune cell therapies. Currently, BRL Medicine has established product pipelines BRL-301 and BRL-303 based on this technology, focusing on hematological malignancies and autoimmune diseases, respectively. Among them, BRL-301 has received IND approval in China for dual indications in Relapsed/Refractory B-cell Acute Lymphoblastic Leukemia (R/R B-ALL) and Relapsed/Refractory B-cell Non-Hodgkin Lymphoma (R/R B-NHL), and is accelerating towards registrational clinical trials. Moving forward, BRL Medicine will rapidly advance next-generation allogeneic universal CAR-T multi-center clinical trials in China and actively explore the application of UCAR-T technology in hematological malignancies, solid tumors, and autoimmune diseases to benefit a broad patient population.