

2025-08-20
In July 2020, BRL Medicine Inc. (referred to as “BRL Medicine”), in collaboration with Xiangya Hospital of Central South University, successfully completed Asia’s first clinical trial using gene editing technology to treat thalassemia. In this clinical study, two transfusion-dependent severe β-thalassemia boys, aged 7 and 8, were successfully freed from transfusion dependence after treatment. Notably, one of the boys (pseudonym "Xixi") became the world’s first patient with severe β0/β0 thalassemia cured by CRISPR gene editing technology—the most severe form of β-thalassemia.
Now, five years later, BRL Medicine announces that this world’s first β0/β0 severe thalassemia patient treated with CRISPR gene editing therapy (BRL Medicine pipeline code: BRL-101) has remained transfusion-independent for over five years. His life has been completely transformed—from lifelong dependence on blood transfusions to living freely. This marks a major milestone in gene editing therapy for inherited blood disorders.

Recent life photo of "Xixi", the first thalassemia child cured by BRL-101
Scientific Breakthrough: Gene Therapy Overcomes the "Hopeless Matching" Dilemma
Thalassemia is an inherited blood disorder caused by globin gene defects leading to impaired hemoglobin synthesis. Patients with severe forms have extremely poor quality of life. Traditional curative methods rely on allogeneic hematopoietic stem cell transplantation, which faces major challenges including high cost, extreme difficulty in matching, and risks such as transplant rejection, graft-versus-host disease (GVHD), and infection.
BRL Medicine’s BRL-101 gene therapy offers new hope. Its mechanism is similar to the world’s first marketed CRISPR therapy, CASGEVY™: using CRISPR gene editing to modify the BCL11A site in the patient’s hematopoietic stem cells. The modified cells are reinfused into the patient, where they self-renew and differentiate to rebuild the modified cell population, thereby treating the blood disorder with the advantage of "one-time treatment, lifelong cure." Moreover, BRL-101 uses electroporation to deliver gene editing materials, avoiding safety issues associated with random insertion by viral vectors. On October 31, 2023, during the FDA’s Cellular, Tissue, and Gene Therapies Advisory Committee meeting discussing CASGEVY™ off-target concerns, two publications by BRL Medicine in Nature Medicine (2019 and 2022) were cited to support the product’s safety.
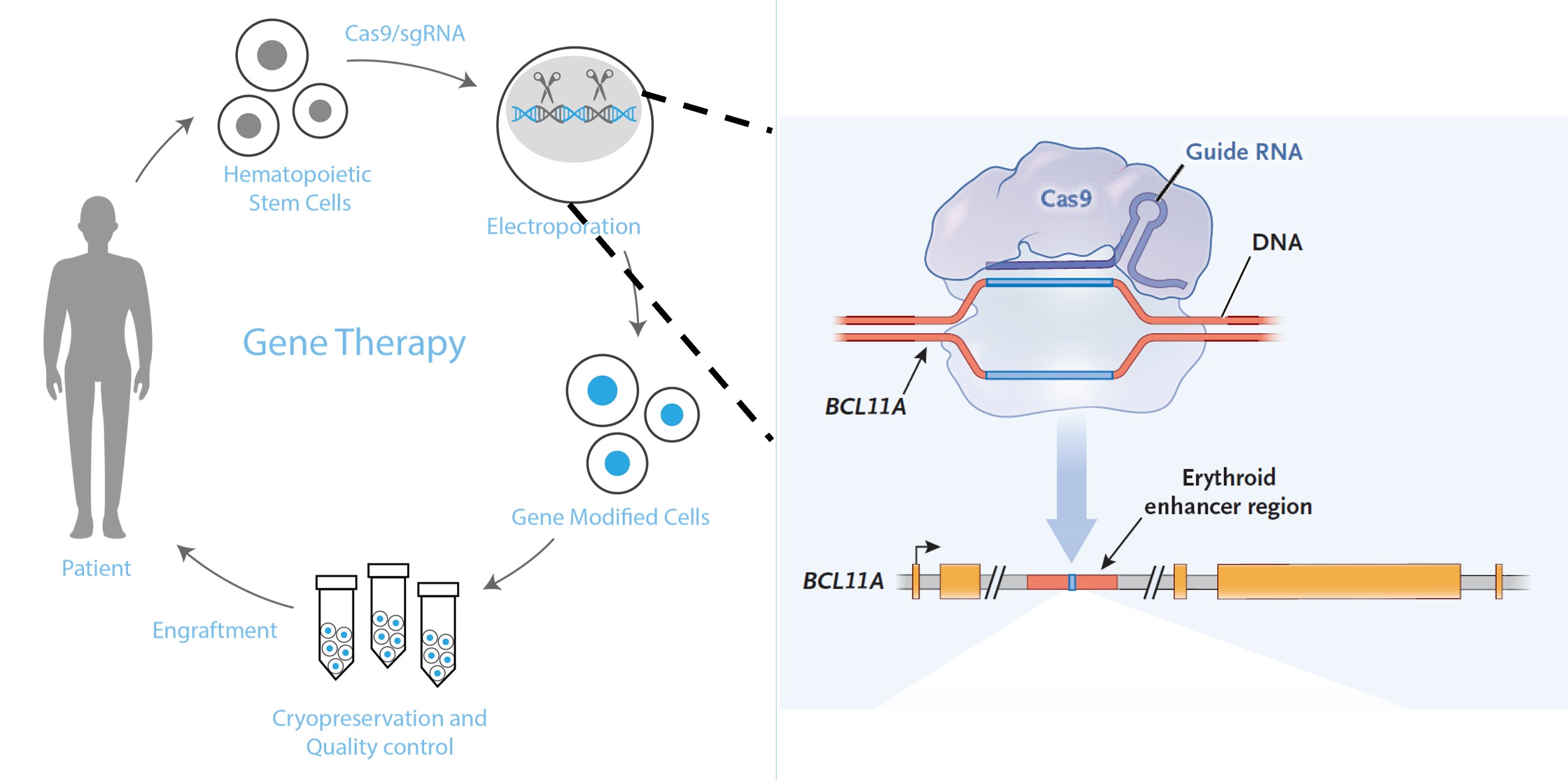
BRL-101 Treatment Strategy
BRL Medicine Gene Therapy Rewrites the "Code of Life"
BRL Medicine has been deeply engaged in the field of gene therapy for many years, with a solid and rapid R&D journey for its first gene therapy product:
Research published in Nature Medicine laid the theoretical foundation for curing inherited blood diseases with gene therapy.
Collaboration with Professor Bin Fu of Xiangya Hospital successfully cured the world’s first child with severe β0/β0 thalassemia; clinical results announced.
Collaboration with Professor Xinhua Zhang of the 923rd Hospital of the PLA Joint Logistics Support Force achieved clinical cures for three thalassemia children in Guangxi.
Paper published in Nature Medicine reported significant efficacy in the first severe thalassemia patient cured for over two years; same year, BRL-101 gene therapy product received IND approval from China NMPA.
Collaboration with Professor Yongrong Lai of the First Affiliated Hospital of Guangxi Medical University completed the cure of China’s first adult severe thalassemia patient, tackling a global treatment challenge.
Completed Phase 1 registered clinical study for BRL-101, curing 15 thalassemia patients globally. Same year, collaboration with Professor Yongrong Lai successfully treated China’s first foreign patient with sickle cell disease (pipeline code: BRL-102).
Completed Phase 2 pivotal registered clinical study for BRL-101, accelerating the product’s path to market.
BRL-101 received China IND in August 2022
Professor Mingyao Liu, Founder of BRL Medicine, stated: "Clinical data fully demonstrate that BRL Medicine’s gene therapy is precisely targeted, safe, highly effective, and durable, significantly reducing the long-term economic burden on patients, making it a more accessible gene therapy. Currently, we are also exploring this therapy for sickle cell disease (pipeline code: BRL-102) and have successfully completed the treatment of China’s first foreign sickle cell patient. In the future, BRL will continue to work with global clinical experts to advance the implementation of this gene therapy, hoping to benefit patients with thalassemia, sickle cell disease, and other inherited blood disorders worldwide as soon as possible."
To date, BRL Medicine’s BRL-101 gene therapy has been selected for presentation at top international conferences including the ASGCT Annual Meeting, EHA Congress, and ASH Annual Meeting due to its breakthrough results. It also won the 2024 Golden Snail Award—the first "Rare Disease Industry Promotion Award" in China’s rare disease field. Related research results have been included in influential reports such as the ZHONGGUO DIZHONGHAI PINXUE LANPISHU (2020) and Payment for Rare Disease Drugs in China Under Common Prosperity (2022).