

2025-06-18
From June 12 to 15, 2025, the 30th Annual Congress of the European Hematology Association (EHA) was grandly held in Milan, Italy. The EHA Congress is the largest international conference in the field of hematology in Europe, bringing together experts and scholars from over 100 countries and regions each year to focus on cutting-edge advances in hematology, present important research data, and discuss the latest challenges and solutions.
On June 15 local time, Dr. Qian Tan, Chief Medical Officer & Chief Operating Officer of BRL Medicine, attended the conference and delivered an oral presentation on the research results of the gene therapy product "BRL-101" for transfusion-dependent β-thalassemia (Abstract Number: S290). Notably, this marks the third consecutive year that BRL Medicine has presented at the EHA Congress. This year, the BRL-101 research abstract stood out among thousands of submissions and was presented as an oral report to showcase the latest clinical progress data to global hematology experts. This indicates that BRL Medicine's innovative products are gradually moving toward global recognition and entering the international stage. BRL Medicine, representing Chinese companies in the cell and gene therapy (CGT) field, is emerging as a world-class new force focused on revolutionary innovative technologies, promoting China's "overtaking on the curve" in the global biopharmaceutical sector.

Chinese Breakthrough on the Global Stage: Showcasing the "Cure Code" Behind Technological Innovation
BRL-101 is a gene therapy product developed based on BRL Medicine's self-developed hematopoietic stem cell platform (ModiHSC®), targeting transfusion-dependent β-thalassemia. ModiHSC® utilizes a gene editing system to genetically modify the patient's hematopoietic stem cells. The modified cells are reinfused into the patient, where they self-renew and differentiate to rebuild the modified cell population, thereby treating the hematological disorder. In July 2020, BRL Medicine, in collaboration with Xiangya Hospital of Central South University, released the world's first clinical data of successful CRISPR gene editing therapy for severe β0/β0 thalassemia. In August 2022, BRL Medicine published a paper in Nature Medicine reporting that the world's first severe thalassemia child treated with BRL-101 gene therapy remained transfusion-independent for over two years, marking a breakthrough in clinical research for domestic gene editing therapy. On August 16 of the same year, the Investigational New Drug (IND) application for BRL-101 was officially approved by the Center for Drug Evaluation (CDE) of China's National Medical Products Administration (NMPA).
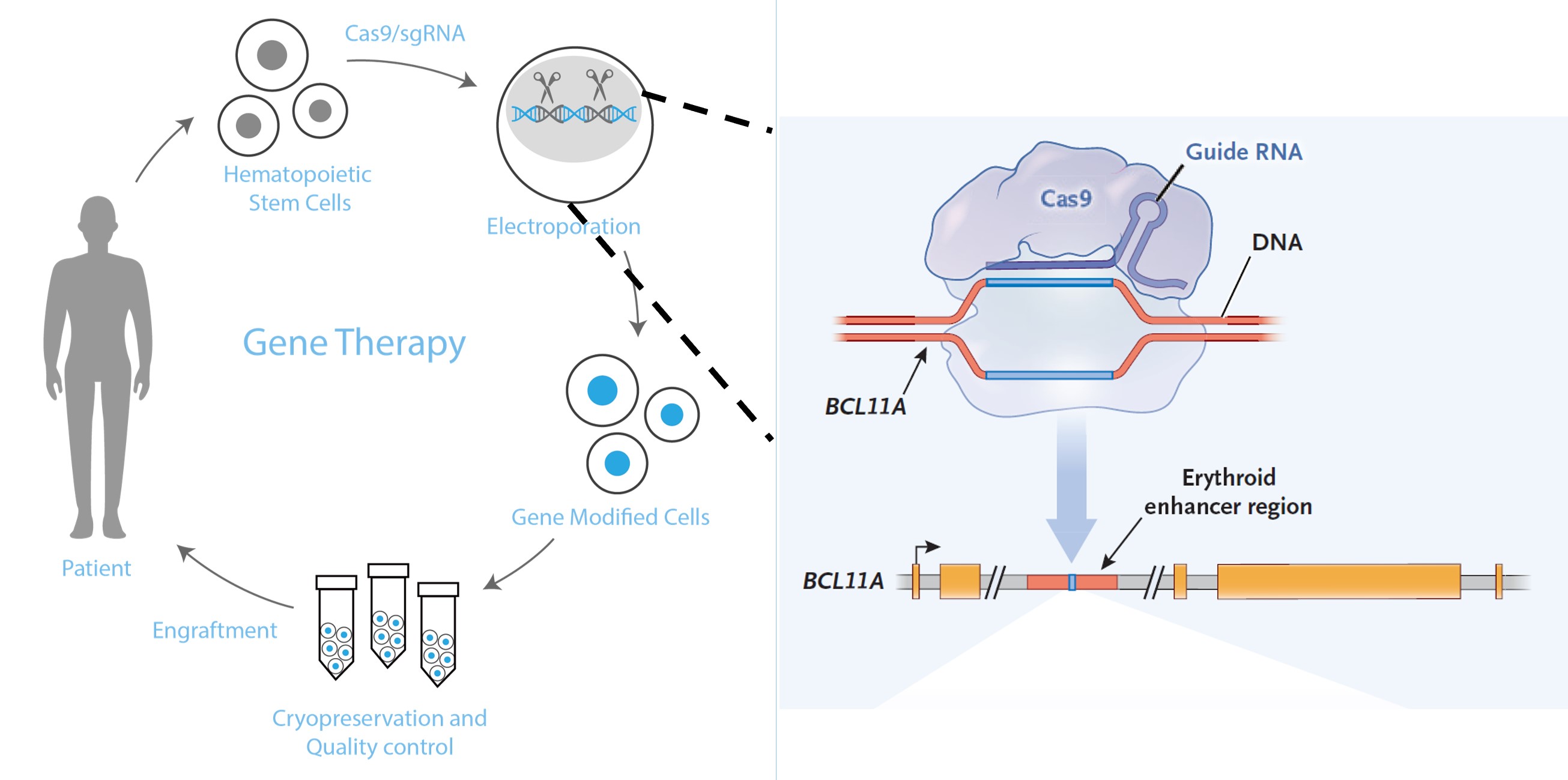
BRL-101 Treatment Strategy
At this conference, Dr. Tan presented exciting long-term follow-up data from BRL-101's early clinical research (IIT) and IND-registered Phase 1 study. This is a study conducted in China to evaluate the "safety and efficacy of autologous hematopoietic stem cell transplantation with γ-globin reactivation for treating severe β-thalassemia." The study enrolled 15 patients aged 6-26 years. Clinical results showed that after receiving genetically edited HSC transplantation, all patients had significantly increased overall Hb and HbF levels. No serious adverse events related to BRL-101 occurred during the entire treatment process, and there were no cases of GVHD, study withdrawal due to adverse events, or death. All adverse events recovered after medical intervention. As of May 23, 2025, the 15 patients who received BRL-101 treatment had a median follow-up time of 25.8 months (range: 14.5-59 months). The longest transfusion independence (TI) duration reached 59 months, indicating that 100% of patients achieved transfusion independence after BRL-101 gene therapy and were all cured.

BRL Medicine's First Adult Thalassemia Patient Treated with Gene Therapy Discharged: Group Photo
Additionally, Dr. Tan presented clinical research results from BRL Medicine's gene therapy for one patient with sickle cell disease (SCD), the first successful case of gene therapy for SCD in China. Clinical results showed that one month after cell reinfusion, the SCD patient demonstrated significant preliminary efficacy: the fetal hemoglobin (HbF) proportion increased from a baseline of 4.6% to 26.1%, and total hemoglobin increased from 52 g/L to 104 g/L without red blood cell transfusion for nearly three weeks. By the sixth month, total hemoglobin continued to increase to 134.1 g/L. No serious adverse events occurred during the entire treatment process. The latest clinical research results presented demonstrate that BRL Medicine's gene therapy offers:
1. Superior Efficacy
All 16 patients (15 with thalassemia and 1 with SCD) who received BRL Medicine's gene therapy achieved 100% efficacy endpoint, successfully achieving a one-time treatment for lifelong cure! Meanwhile, the average total hemoglobin level of patients exceeded 120 g/L, rapidly recovering to healthy levels, with efficacy significantly superior to marketed international products and domestic products under research.
2. Enhanced Safety
BRL Medicine's gene therapy product uses electroporation to deliver gene editing materials, avoiding safety issues associated with random insertion by viral vector-based cell and gene therapies. Meanwhile, the gene editing target has undergone extensive off-target studies, proving no off-target effects and demonstrating high safety.
Overall, compared to other gene therapies, BRL Medicine's gene therapy is more efficient, convenient, and safe, with advantages such as excellent targeting, high safety, broad applicability, and significant therapeutic effects. It achieves a one-time treatment for lifelong cure and is expected to become a more accessible therapy for the public. Currently, BRL-101 is undergoing a pivotal Phase 2 registered clinical study, with the first patient having achieved "transfusion independence" for nearly 5 years. In the future, BRL Medicine will continue to work with clinical experts to fully advance the translation and implementation of this clinical research, hoping to benefit global patients with thalassemia and SCD as soon as possible.